
- #SMALL PROTEIN SCAFFOLD BETTER THAN ANTIBODY FULL#
- #SMALL PROTEIN SCAFFOLD BETTER THAN ANTIBODY FREE#
Since then, she says it’s been “a roller-coaster ride of personal growth” as she has navigated the vastly different terrains of the U.S. It’s now been 12 years since Cobb started the company, which develops treatments for fibrotic (i.e., scarring) diseases and is listed on the Australian Securities Exchange (ASX). “They never expected us to last past our first 12 months of funding.” That’s what Sam Cobb, managing director and CEO of Australian biotech AdAlta, recalls some of the original academic shareholders telling her years after the company had succeeded.
#SMALL PROTEIN SCAFFOLD BETTER THAN ANTIBODY FREE#
This construct self-assembled and when added to T cell culture could stimulate T cell growth to the same degree as free CD28 antibody, indicating its function.By Dan Schell, Editorial Director, Life Science Leader The authors engineered a 6-loop cTRP construct with a single-chain Fv domain capable of binding CD28. T cell stimulation and growth in vitro requires co-stimulation from its TCR and co-receptors like CD28.
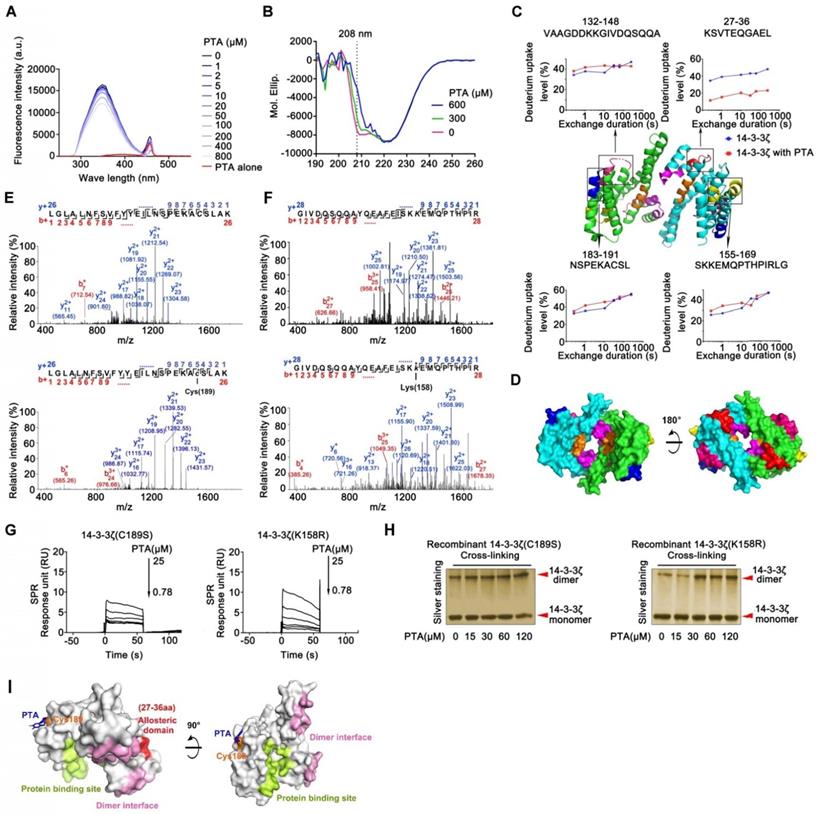
This tetrameric cTRP-MHC complex stained CMV-specific T cells to a similar degree to traditional tetramer reagents. The authors used their 6-loop construct to produce cTRP tetramers conjugated to four peptide-MHC complexes presenting a peptide derived from cytomegalovirus (CMV). MHC tetramers are useful immunologic reagents used to identify and stain T cells specific for a certain peptide-MHC complex.

Constructs containing peptide-binding or protein ligation domains were able to successfully bind their respective partners, allowing flexibility in loading many cargo types onto cTRP24. Numerous protein cargo types were tested: peptide-binding domains, protein ligation domains, fluorescent proteins, as well as single-chain MHC molecules and single-chain Fv or receptor domains targeting important immune modulators. Next, the authors wanted to test if cargo could be bound to cTRP24 and retain function. Additionally, the authors used a mammalian expression system to broaden their ability to test modifications to cTRP24 that might not be well expressed in bacterial systems. Both the 12-loop and 6-loop constructs were stabilized by this modification and could self-assemble into dimers or tetramers, respectively. The authors sought to stabilize the interaction by modifying the protein termini to contain cysteines allowing disulfide bonds between multimers.
#SMALL PROTEIN SCAFFOLD BETTER THAN ANTIBODY FULL#
Only the 12-loop construct could assemble into a dimer of the full 24 looped structure, however dimerization was not complete. The authors modified their construct to express repeats of 3, 4, 6, or 12 loops to see if these smaller units could self-assemble into the full cTRP of 24 repeats. Shaped like a donut, cTRP24 has an outer diameter of approximately 100 angstroms and inner diameter of approximately 60 angstroms. The authors computationally designed and expressed a cTRP with 24 tandem loop repeats, named cTRP24. The results of their engineering efforts were recently published in Nature Structural & Molecular Biology. The Bradley, Stoddard, Riddell and Kaiser labs teamed up to further develop cTRPs with functional domains. Circular TRPs (cTRPs) can be made from repeating motifs, allowing protein cargo to be attached at regular intervals around the structure. Additionally, TRPs can be conjugated to various forms of functional cargo at defined positions. TRPs are useful for protein engineering as they are compact, have high thermal stability, good solubility characteristics, and are relatively easy to design and express.
Tandem repeat proteins (TRPs) are small proteins with repeated sequences. Protein engineering offers unique solutions for protein scaffolding for a variety of uses. Viruses, Vaccines and Infectious Diseases.Institutional Partners & Collaborations.Vaccine and Infectious Disease Division.Subscribe to Oncology Insights Newsletter.


 0 kommentar(er)
0 kommentar(er)
